
Density is the amount of flux passing through a specific region perpendicular to the direction of the flux.

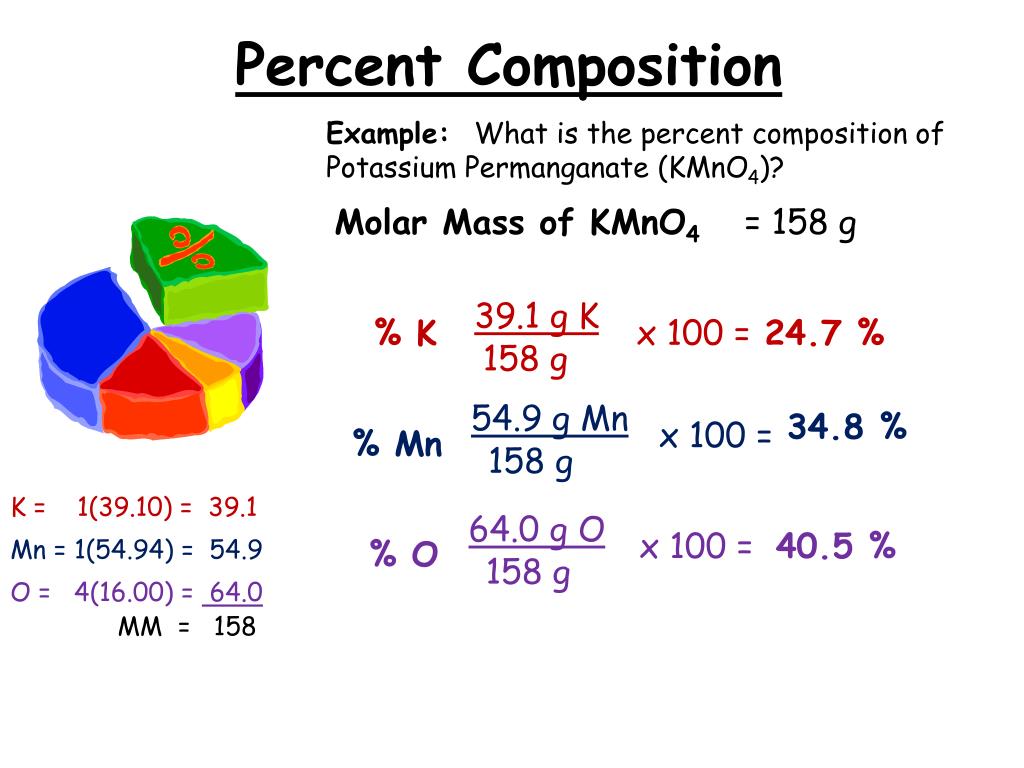
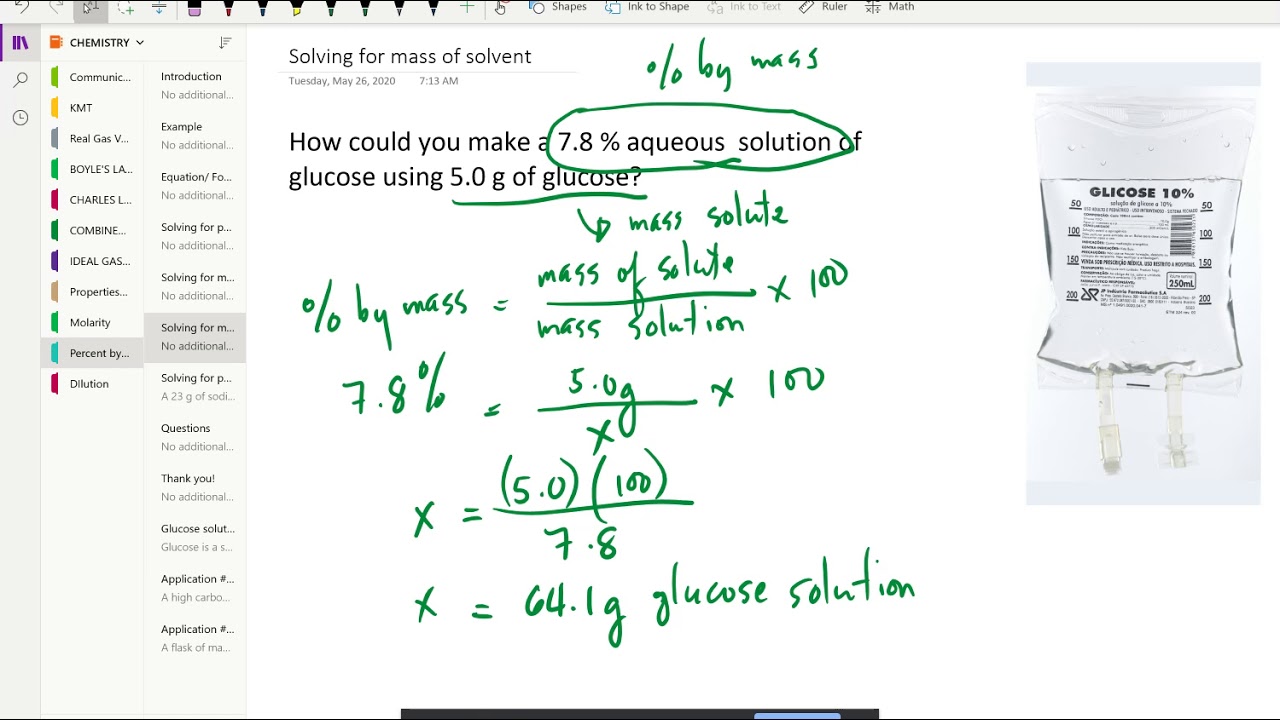
You’ll determine the mass percentage of every element with these masses. Since this type of concentration is expressed as a percentage, the indicated proportion must be multiplied by 100 is calculated using Mass/Volume Percent. Mass percent (component’s mass total mass) x 100 or Percentage of mass (solute’s mass mass of solution) x 100 The Mass percent formula is expressed as solving for the molar mass also for the mass of every element in 1 mole of the compound.
#Formula for percent by mmass free
Electric flux density is a measurement of the strength of an electric field generated by a free electric charge, which corresponds to the number of electric lines of force travelling across a particular area. The Mass Volume percent formula is defined as the ratio of the mass of solute that is present in a solution, relative to the volume of the solution, as a whole.Where, F denotes flux and A denotes a cross-sectional area Magnetic flux density and electric flux density are involved.When discussing flux density formulas, we must be rather specific because there are typically two flux density formulas.JEE Main 2022 Question Paper Live Discussion.Difference Between Selling And Marketing.TS Grewal Solutions Class 11 Accountancy.TS Grewal Solutions Class 12 Accountancy.CBSE Previous Year Question Papers Class 12.CBSE Previous Year Question Papers Class 10.To express the value as a percentage, you need to multiply by 100 at the top. Mass percent (mass of chemical / total mass of compound) x 100 is the formula for the mass percent of a compound. Step 1: Define a formula for calculating a compound’s mass percent. NCERT Solutions For Class 6 Social Science Finding the Mass Percentage in Simple Steps.NCERT Solutions for Class 7 Social Science.NCERT Solutions for Class 8 Social Science.So the molecular formula of cyclopropane is C 3H 6.Īuthor: Fred Senese Chemistry Online! How can I find the molecular formula of a gas from experimental data?Ĭomments & questions to Revised 02/23/18. In this case, the molecular formula weight divided by the empirical formula weight is 42.0 20768/14.026 94 = 3. Is repeated to make the molecular formula. This number tells you how many times the empirical formula Divide the molecular weight by the formula weight.Find the empirical formula weight by adding up the weights of the atoms in the empirical formula.įor CH 2, the formula weight is 12.011 + 2 × 1.00797 = 14.026 94 g/mol.

These are the subscripts in the empirical formula.


 0 kommentar(er)
0 kommentar(er)
